bag-CPAP system +connections & tubing, +mask S, s.u. 472851
Valid Article
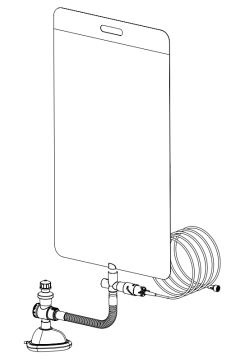
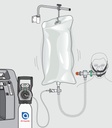
bag-CPAP system
Definition
The Bag-CPAP is a disposable single patient use non-invasive ventilation device intended for patients requiring Continuous Positive Airway Pressure and moderate or high oxygenation in a hospital environment or in an emergency situation. The Bag-CPAP is intended for use on adult patients (weight > 40 kg).
Specifications
The device is composed by 4 main parts:
- 1) Downstream body: This part is used to channel the oxygen or air-oxygen mixture from the system to the patient and it's composed by:
- a) A 22 male patient port used to connect the patient interface.
- b) A main body.
- c) An anti-asphyxia valve which allows the patient to breathe ambient air in case the device is stuck.
- d) A one-way valve that prevents the patient from rebreathing his own CO2.
- e) A 22 male connector that fits the patient circuit.
- 2) Upstream body: This part is used to connect the oxygen source and the patient connector and it's composed by:
- a) A 22 male connector that fits the venturi system.
- b) A custom connector for the oxygen reservoir.
- c) A 22 male connector for the patient circuit
- d) A “blind” 22 female connector used to close the patient port and let the reservoir to inflate.
- 3) Venturi system: This component is made by two parts, an upper body that moves up and down along 3 guides in the bottom part. Thanks to this motion, the operator will be able to activate or deactivate the venturi effect depending on the therapy chosen. When the venturi effect is switched off, pure oxygen will be delivered to the patient at the flow selected on the source; when it's switched on, the system mixes the oxygen with ambient air and allows to increase the input flow, decreasing the FiO2 .
- 4) PEEP valve: This component consists of a valve that regulates the extrinsic end-expiratory pressure in the lungs, preventing airway collapse. The pressure can be adjusted to better suit the chosen therapy.
Technical specifications
- Delivered oxygen concentration:
- >90% with 15 l/min (venturi OFF)
- 50% with 5 l/min (venturi ON)
- Volume of oxygen bag: 30 liters
- Patient circuit length: 1.8 m
- Device weight: 350 gr
- Patient port connection: Male 22 mm
- Useful life: 7 days
- Operating temperature: from -18°C to +50°C
- The product is single use and single patient.
Packaging & Labelling
IFU in each pack
Instructions for use
Precautions for Use
Contraindications
- Multiple organ failure (i.e. another organ failure associated with the respiratory distress)
- Altered level of consciousness (GCS <9 or P/U on the AVPU scale)
- Uncontrolled hemodynamic instability (severe shock, massive doses of vasopressors, ,..)
- Noncompliance of the patient (due to comprehension difficulties or to stress and a choking impression when the face mask is applied. The latter can be significantly reduced by accompanying the patients with step by step explanations as the procedure is put in place).
- Non drained pneumothorax or compressive hemothorax
- Vomiting, gastrointestinal, airway, or facial bleeding, upper airway obstruction.
Maintenance
Do not sterilize
MSF requirements
The Bag CPAP device was developed by Air Liquide by request from MSF after feedback of challenges related to CPAP O-two use in the field. Specifically MSF asked Air Liquide to make something that is good for low resource setting, for use with the MSF STD oxygen concentrator, that can be used for long durations multiple times (single patient), and has consistent pressure. The Bag-CPAP device has CE approval, so is available in European market. It is also cheaper than CPAP O-two device.
The Bag-CPAP device as tested in DRC by OCP with positive field feedback and easy to use.

![[EEMDCONE12-] CONCENTRATOR O2 (DeVilbiss 1025KS) 10l, 220V + access.](/web/image/product.template/573846/image_256/%5BEEMDCONE12-%5D%20CONCENTRATOR%20O2%20%28DeVilbiss%201025KS%29%2010l%2C%20220V%20%2B%20access.?unique=089f179)
![[EEMDCONE4--] CONCENTRATOR O2 (DeVilbiss 525KS) 5l, 220V + access.](/web/image/product.template/570014/image_256/%5BEEMDCONE4--%5D%20CONCENTRATOR%20O2%20%28DeVilbiss%20525KS%29%205l%2C%20220V%20%2B%20access.?unique=089f179)
![[SCTDCPAP202] CPAP O-Two single use SET, mask n°3 child, + manometer](/web/image/product.template/574008/image_256/%5BSCTDCPAP202%5D%20CPAP%20O-Two%20single%20use%20SET%2C%20mask%20n%C2%B03%20child%2C%20%2B%20manometer?unique=089f179)